Abstract
Introduction
Achieving a PET-negative metabolic (m)CR with salvage chemotherapy prior to autologous stem cell transplantation (ASCT) in relapsed/refractory Hodgkin lymphoma (R/R HL) is a strong predictive factor for long-term progression-free survival (PFS). In a phase I dose-escalation trial we have shown a favorable toxicity profile of 3 cycles of DHAP in combination with brentuximab vedotin (BV) with a high mCR rate (12/12 patients). A subsequent phase II study in 55 patients has been performed; the 6 patients treated at full dose of BV-DHAP in the phase I part were added to the current analysis.
Methods
BV was given at a fixed dose of 1.8 mg/kg on day (d) 1 of 3 cycles of DHAP q 3 weeks (dexamethasone 40 mg iv d 1-4, cisplatin 100 mg/m² iv d1 and cytarabine 2x2 g/m² iv d2). Cycle 2 was used for stem cell mobilization; after the 1st and 3rd cycle patients received pegylated G-CSF to prevent prolonged neutropenia and dose delays. The primary endpoint of the study was the mCR rate after 3 cycles of BV-DHAP based on central PET-review. Patients with a partial or complete response proceeded to high dose chemotherapy (BEAM) and ASCT.
Results
Twenty-three of the 61 patients (29 males; median age 29 yrs, range 19-71) had not achieved a CR to 1st line treatment (37%), which consisted of ABVD (n=45), (escalated) BEACOPP (n=11) or other regimens (n=5). The median time from response to 1st line treatment to relapse was 6 months (range 0-160 months); 38 patients (62%) had either primary refractory disease or early relapse (<1 year).
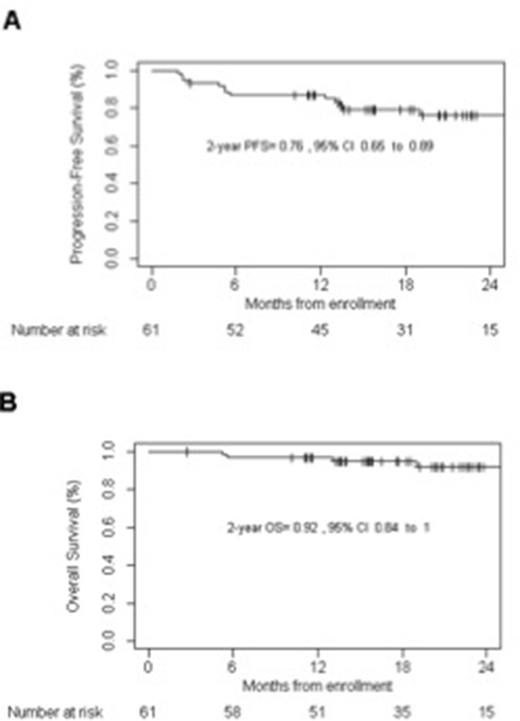
All patients received the 1st cycle of BV-DHAP, 90% completed all 3 cycles and 87% underwent ASCT. G-CSF mobilized stem cells were harvested successfully after the 2nd cycle with 1 apheresis in all patients (median yield: 6x106 CD34+/kg; range: 2-23). Based on intention to treat analysis 48 patients achieved a metabolic CR after 3x BV-DHAP (mCR rate 79% (n=48/61)), 5 patients had a metabolic PR (8%) and 4 had PD (6.5%). Four patients went off study before the first evaluation (1 PD after cycle 1, 1 liver function abnormalities after cycle 2, 2 withdrew because of emotional problems). After ASCT 4 out of 5 patients with mPR converted to an mCR. At a median follow-up of 20.9 months the 2-year PFS is 76% (95% CI 65-89), and the OS projected at 2 years is 92% (95% CI 84-100).
Hematologic toxicity after BV-DHAP consisted of neutropenia grade 3/4 in up to 63% of patients and thrombocytopenia grade 3/4 in up to 81% of patients. Only 3 patients had a bleeding event (epistaxis (n=2) and menorrhagia (n=1), all recovered without sequelae after platelet transfusion). Febrile neutropenia or infections after BV-DHAP occurred in 18 patients (15 grade 3 and 3 grade 4). A total of 21 SAE's other than fever/infections were reported, with kidney dysfunction (n=4; most likely related to cisplatin), malaise (n=3), nausea/vomiting (n=3) and liver function abnormalities (n=3) occurring in 3 or more patients. 11 patients had peripheral neuropathy (PN) already at baseline (10 grade 1, 1 grade 2); worsening of PN was not observed in those patients. New onset PN was seen in 19 patients (15 grade 1, 4 grade 2); no grade 3/4 PN was observed.
Three deaths occurred during the study period, 1 encephalitis (exact cause unknown), 1 veno-occlusive disease, both after BEAM/ASCT and 1 trauma capitis (unrelated). One patient who refused further treatment after cycle 1 and went off study died of progressive disease.
Conclusions
Salvage immunochemotherapy with 3 cycles of BV-DHAP induces a high mCR rate of 79% in patients with R/R HL, which is an optimal starting point for consolidation with BEAM and ASCT and may contribute to a higher cure rate. The toxicity of combining BV and DHAP is acceptable and mainly consists of manageable hematologic toxicity and fever/infections.
Hagenbeek:Millennium/Takeda: Consultancy, Honoraria, Research Funding. Morschhauser:Janssen: Other: Scientific Lectures; BMS: Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lugtenburg:Millennium/Takeda: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Roche: Consultancy; BMS: Consultancy; Sandoz: Consultancy; Genmab: Consultancy. Gastinne:Millennium/Takeda: Honoraria. Tonino:Takeda: Consultancy, Honoraria. Kersten:Millennium/Takeda: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Gilead: Honoraria; Kite Pharma: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal